Research projects
(1)Analysis of EphA2 processing by MT1-MMP in the molecular mechanisms of malignant progression of cancer and clinical application for cancer therapy and diagnosis.
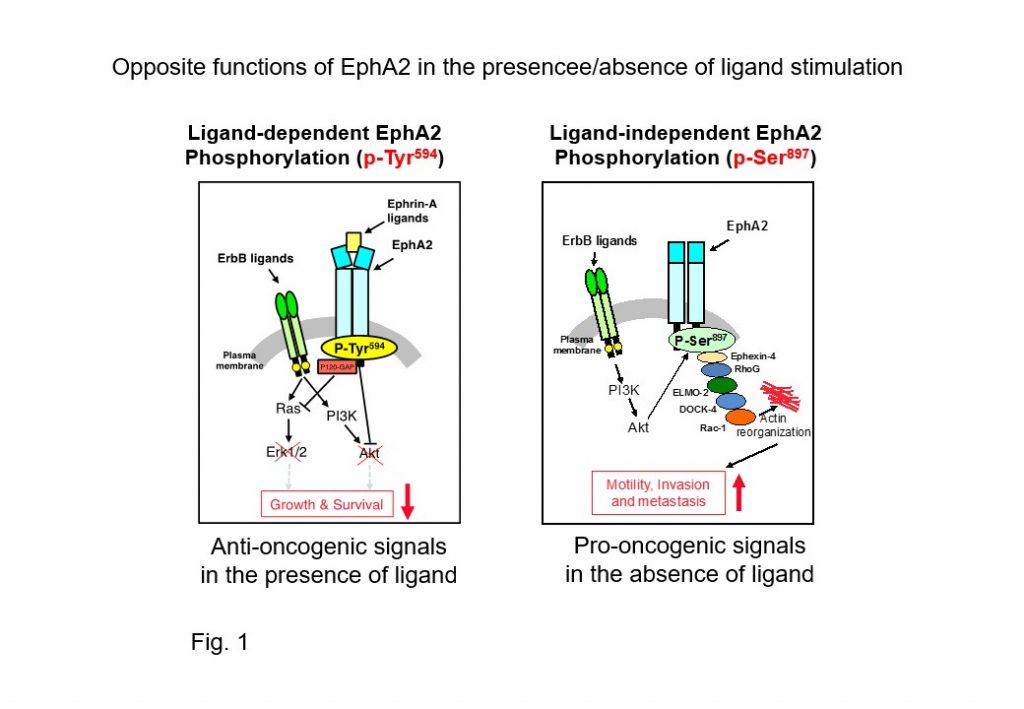
Erythropoietin producing hepatoma receptor-A2 (EphA2) is a target molecule for cancer therapy because it is expressed at high levels in malignant tumor cells and tissues, and its expression is significantly associated with the malignant progression of tumors.
To date, many clinical trials targeting EphA2 have been conducted using inhibitory compounds of tyrosine phosphorylation, but effective tumor suppressive activity has not yet been reported. This may be caused by EphA2 regulating opposing pro-oncogenic and anti-oncogenic signal transmitters in cancer cells. Unlike the EGF receptor (EGFR), which is a pro-oncogenic signaling transmitter, EphA2 indeed acts on tumor suppression in the presence of ligands through the ligand-dependent phosphorylation (pY594) of tyrosine residues (Fig. 1 right). In the absence of ligands, EphA2 acts in collaboration with EGFR and its down-stream signals to induce serine residue phosphorylation (pS897), promoting cancer progression (Fig. 1 left). Although these opposing functions of EphA2 were thought to be regulated by the presence or absence of its ligand expression, it has recently been shown that a soluble EphA2 ligand, Ephrin-A, is abundantly produced in tumors and normal stromal tissues, particularly in cancer sera. Therefore, evasion from ligand-dependent tumor suppressor signals by ubiquitously expressed Ephrin-A in vivo is essential for tumor cells to acquire malignant phenotypes, and understanding these molecular mechanisms will provide new opportunities for molecular therapies targeting EphA2.
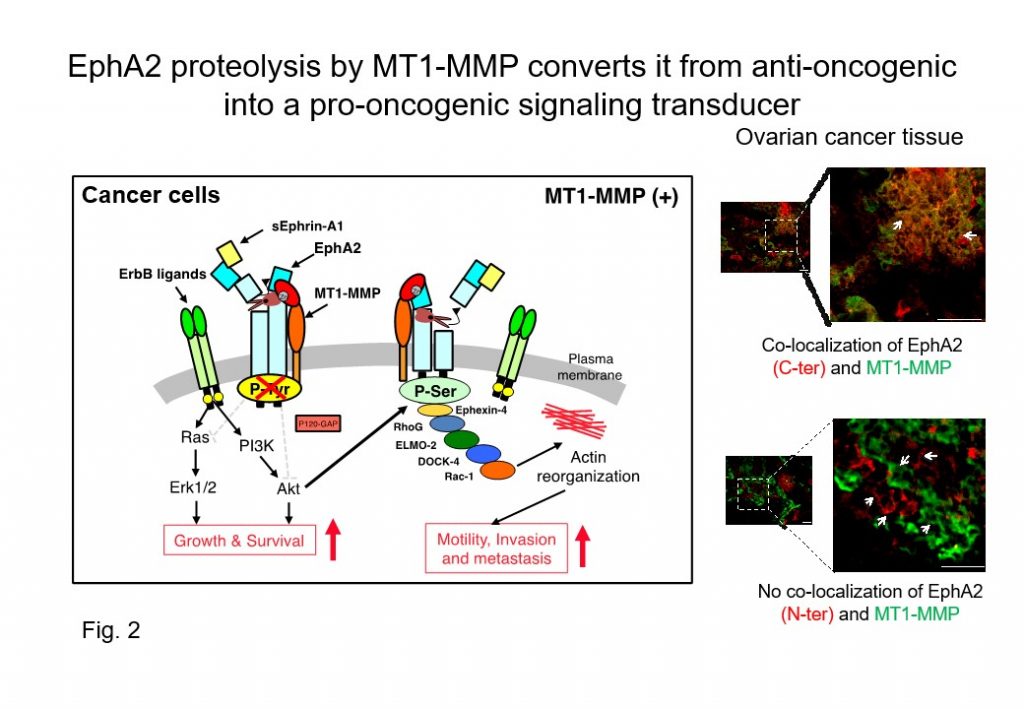
We have previously demonstrated that i) MT1-MMP interacts with EphA2 at the cancer cell surface (Cancer Sci 2008), ii) cleavage (proteolytic processing) of the ligand-binding domain at the N-terminus converts EphA2 into an N-terminally truncated EphA2 fragment lacking the ligand-binding site (Cancer Res 2015), and iii) N-terminal EphA2 fragments are released and iv) detected at high levels in sera from patients with certain cancers (Cell Death & Diseases 2017).
These results suggest that the N-terminal-truncated EphA2 fragments are stably expressed at the cancer cell surface, and act as a transmitter of pro-oncogenic signaling (Fig. 2). Moreover, the N-terminal EphA2 fragment that is released from cancer cells might be a candidate biomarker for cancer diagnostics.
Therefore, we are developing unique drug discovery and cancer diagnosis methods based on the molecular basis of the malignant progression of EphA2 fragments produced by MT1-MMP proteolysis.
(2) Development of a cancer diagnostic biomarker using laminin γ2 monomer chain expressed specifically in malignant cells
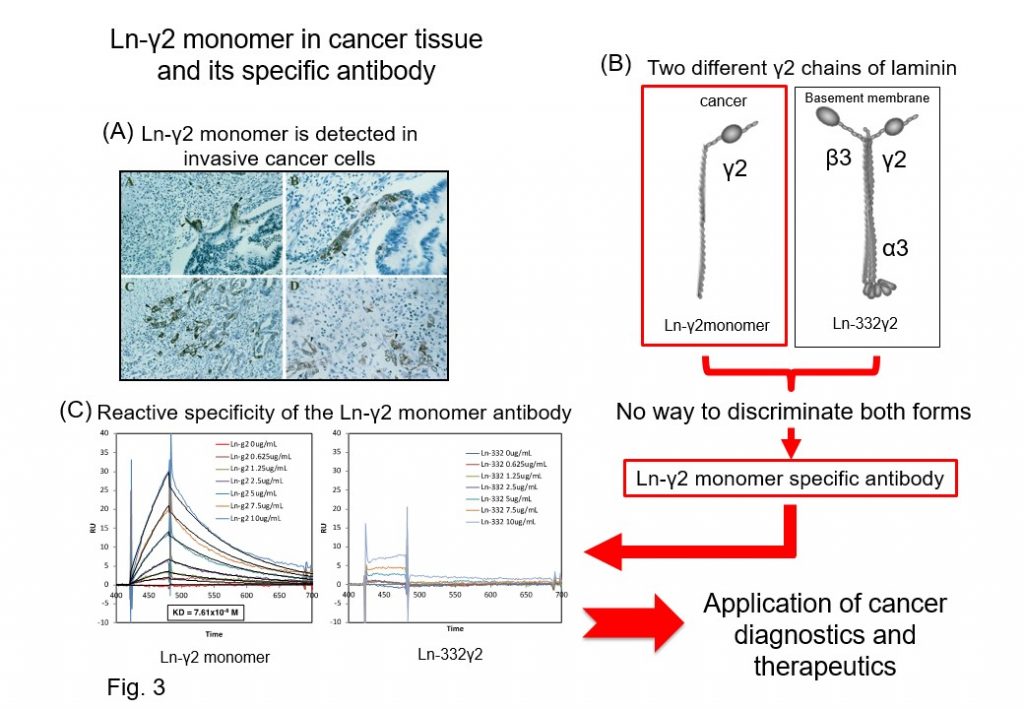
The Laminin γ2 monomer chain (Ln-γ2 monomer) was identified as an extracellular matrix specifically expressed in malignant cancer cells and tissues by our group (Cancer Res 1999) (Fig. 3A and B). Through proteolytic processing of the Ln-γ2 monomer chain by MT1-MMP, the Ln-γ2 monomer liberates EGF-like fragments that act as ligands for EGF receptors and can promote cancer malignant progression through activation of EGF receptors and down-stream signaling in cancer cells. Hence, the Ln-γ2 monomer was believed to be a potential new biomarker of invasive cancers at the time of its discovery, but it did not reach clinical application. The major reason was that the Ln-γ2 monomer gene is identical to that of Ln-332γ2, which forms basement membranes, and there was no antibody that was able to recognize the Ln-γ2 monomer protein selectively, which is essential for clinical application. To solve this problem, we established specific antibodies that can selectively recognize the Ln-γ2 monomer chain but not Ln-332 γ2 chains (Cancer Res 2008, 2016) (Fig. 3C).
We next applied Ln-γ2 monomer specific antibodies to cancer diagnosis using body fluids, and found high levels of Ln-γ2 monomer in urine from patients with bladder cancer, including early-stage cancers (Cancer Sci 2015, BMC Biomarker Res 2018). Moreover, by testing serum specimens from patients with hepatocellular carcinoma, we found that serum Ln-γ2 monomer might be useful for hepatocellular carcinoma diagnosis (Cancer Sci 2017). We are currently accumulating further evidence for Ln-γ2 monomer as a diagnostic and prognostic biomarker of hepatocellular carcinoma through basic and clinical research using clinical specimens and transgenic animals.
(3) Mathematical modeling for the EphA2 and EGF-receptor and their downstream signal pathways involved in the malignant progression of hepatocellular carcinoma
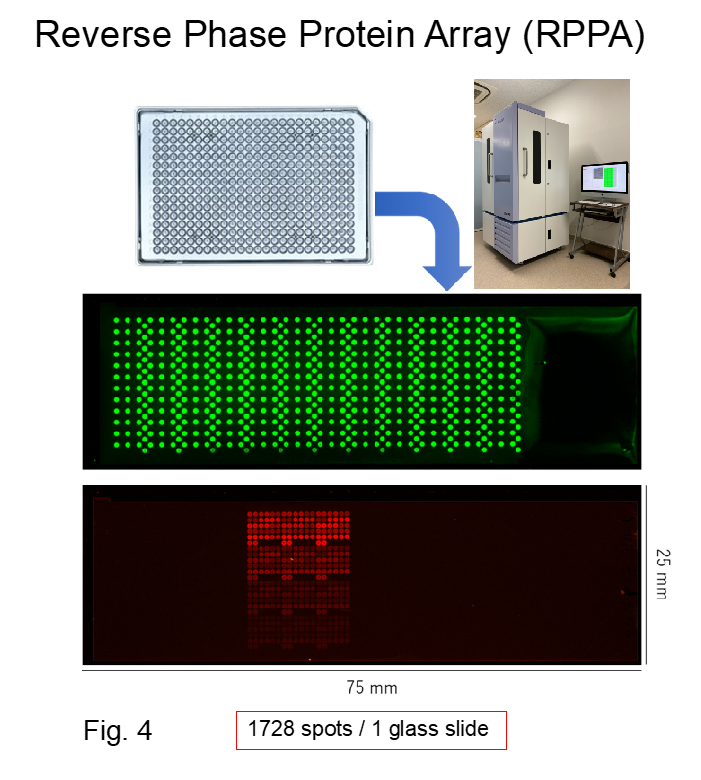
In this study, we analyzed EphA2 and EGFR signal pathways that are cross-talked with each other and involved in the malignant progression of hepatocellular carcinoma using mathematical modeling. First, we collected quantitative information regarding the detailed activation and expression of EphA2 and EGFR and their downstream molecules, over time, in hepatocellular carcinoma cells that do not contain driver gene mutations in the presence/absence of EphA2 processing using reverse phase protein array (Fig. 4). We then constructed simple mathematical models using these data for analyzing the whole picture for EphA2, EGFR, and their downstream signals, and tried to visualize and predict key pathways and molecules that play a role in malignant progression of hepatocellular carcinoma by mathematical simulation. Finally, we simulated the signaling cross-talk between them that was involved in the malignant progression of hepatocellular carcinoma to identify novel pathway(s) and molecule(s) as possible targets for the development of novel cancer therapies and diagnostics.